EXO-Sugar-MAP
 Glycals are unsaturated sugar derivatives constituting a large family of polyhydroxylated chiral synthons. The endo-glycals are widely exploited in the synthesis of natural products and for biochemical applications. In contrast, the chemistry and biology of exo-glycals remained unexplored until recently. Their synthetic chemistry suffers from some limitations in terms of substitution of the double bond, and the control of their stereochemistry is challenging.
Glycals are unsaturated sugar derivatives constituting a large family of polyhydroxylated chiral synthons. The endo-glycals are widely exploited in the synthesis of natural products and for biochemical applications. In contrast, the chemistry and biology of exo-glycals remained unexplored until recently. Their synthetic chemistry suffers from some limitations in terms of substitution of the double bond, and the control of their stereochemistry is challenging.
Since the release of Boromycin and Bortezomib drugs, borylated compounds have also stimulated the pharmaceutical industry’s interest for various applications, and we became interested in the chemistry of borylated exo-glycals.
The spirit of this ARC consortium is thus to gather a variety of expertise and create a synergy in the fields of synthetic organic chemistry both experimental and computational:
Research Line 1 mentored by Prof. Raphaël Robiette, UCLouvain, in physical organic chemistry; Research Line 2 developed by Prof. Berionni, UNamur and in carbohydrate chemistry and biochemistry Research Line 3, supervised by Pro. Stéphane Vincent, UNamur.
Research line 1: synthetic methodologies
Objective 1: Development of olefination methodologies toward borylated exo-glycals.
Objective 2: Development of a synthetic strategy toward borylated exo-glycals via boryl-Heck reactions.
The boron-Wittig reaction
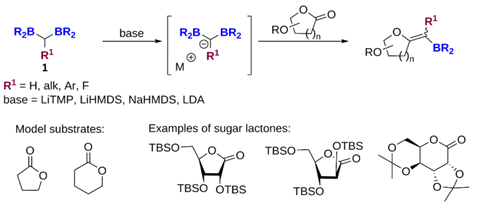
We are investigating the possibility of synthesizing borylated exo-glycals using the boron-Wittig reaction of gem-diboryl derivatives. We started our investigations by considering the reaction of bis[(pinacolato)-boryl]methane (1, R1 = H; R2 = pinacol)[1] with model lactones in order to find suitable reaction conditions for the desired transformation (Scheme 1).
 |
||
|
Scheme 1 – Development of the boron-Wittig reaction for the synthesis of borylated exo-glycals (n = 1, 2) |
The Peterson reaction
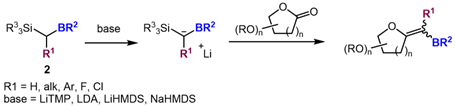
Several examples of C-borylated silane involved in Peterson reactions have been reported in the literature. However, a potential competition between the silyl elimination and the boryl elimination has been shown. Our investigations start by considering the reaction of trimethylsilyl(pinacolato)-borylmethane (2, R1 = H; R2 = pinacol; R3 = Me)[2] with model aldehydes and ketones in order to elucidate the chemoselectivity of the process. The reaction will then be optimized with lactones substrates (scheme 2).
 |
||
|
Scheme 2 – Development of the Peterson reaction for the synthesis of borylated exo-glycals (n = 1, 2) |
The boron-Heck reaction
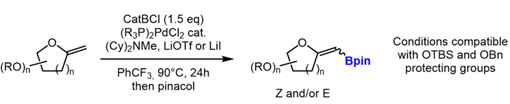
The formal boryl-Heck reaction of non-cyclic alkenes with chloro-catechol borane has been used by Watson for the synthesis of vinyl pinacol boronates.[3] This palladium-catalyzed reaction could be potentially extrapolated to exo-glycals,[4] and open the way towards tetra-substituted exo-glycals via a Suzuki-Miyaura coupling.
 |
||
| Scheme 3 - Pd-catalyzed Boryl-Heck reactions of exo-glycals (n = 1,2) |
[1] For the synthesis of 1, see: J. R. Coombs, L. Zhang, J. P. Morken, J. Am. Chem. Soc. 2014, 136, 16140.
[2] Matteson, D. S.; Majumdar, D. Organometallics 1983, 2, 2, 230-236.
[3] For a recent review on Boryl-Heck reactions, see: Vulovic, B.; Watson, D. A. Eur. J. Org. Chem. 2017, 4996.
[4] Idowu, O.O.; Hayes, J.C.; Reif, W. B.; Watson, D.A. Org. Lett. 2021, 23, 12, 4838-4842.
Research line 2: Physical organic chemistry
One of the most powerful tools for predicting a molecule's reactivity profile is understanding the mechanisms correlated with a comparative analysis of the reaction on a variety of substrates. Quantitative reactivity maps have been created for important
classes of nucleophiles and electrophiles and are crucial in the development of synthetic pathways. A reactivity mapping approach has never been realized in exo and endo-glycal chemistry despite its broad usefulness. Our goal is to develop such a predictive tool which will be of paramount importance in the design of new reactions leading to stereoselective generation of polysubstituted glycals.
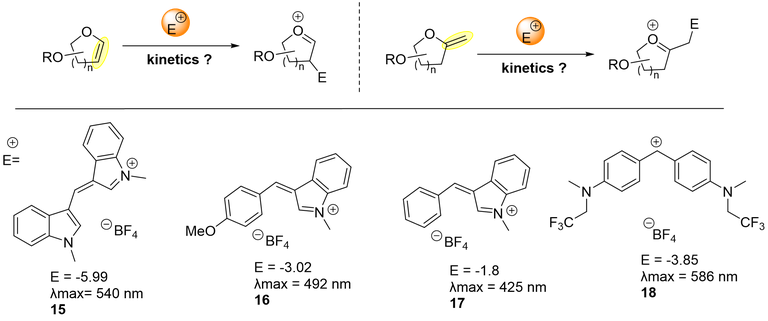
This part of the project is devoted to the systematic characterization of the impact of the exo and endo-glycals substitution pattern on the nucleophilicity of their C=C bond. Bio-relevant electrophiles such as iminium ions and carbocations will be used to determine quantitatively the nucleophilicity of these cyclic enol-ethers for the first time.
This work will focus on the measurement of nucleophilicity of the C=C bond of cyclic enol ethers and of endo and exo-glycals towards a series of electrophiles.
 |
||
| Scheme 1: Model explanation |
To do so, Equation characterizes electrophiles by one parameter, E, and nucleophiles by the solvent-dependent nucleophilicity parameter N and the sensitivity parameter sN and k2 is the second-order rate constant (in M–1 s–1) at 20 °C. Determining kobs is detailed in the scheme below (Scheme 1).
r = kobs [E] with kobs = k2 [N] (Eq. 1)
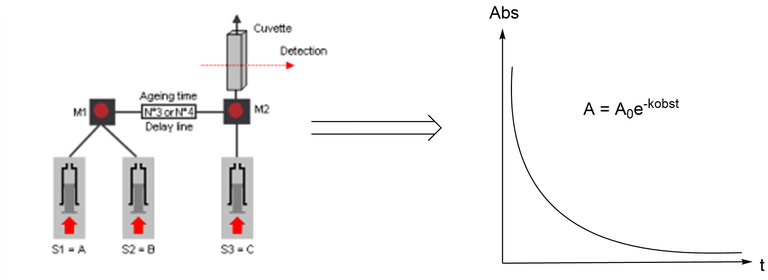
The reaction rates at given [Nu]/[E] will be measured by monitoring the decay of its UV-Vis absorbance by a stopped-flow spectrophotometer in absorbance mode. Variation of the nucleophilic species concentrations will allow determining the reactions rate constant k2 for each electrophile.
 |
||
| Scheme 2: Stopped-flow configuration and expected mono-exponential decrease of the absorbance of the electrophile confirming a pseudo-first order reaction. |
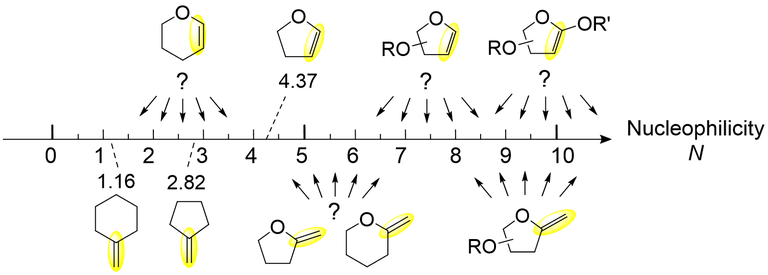
The Mayr and Patz equation will be employed for determining the nucleophilicity of a representative set of glycals and for ranking them in the nucleophilic reactivity scale. Using excess of nucleophilic species will create a pseudo first order reaction toward the electrophile (Eq. 1) allowing to determine the second order rate constant k2.
log k2 (20 °C) = sN(N + E) (Eq. 2)
Using Equation 2 and testing our nucleophile with different electrophiles will allow us to determine N and therefore create a reactivity scale.
 |
||
|
Scheme 3: Representative enol ethers and other nucleophiles with a C=C (some already reported in the Mayr nucleophilicity scale) and those under investigation. |
Research line 3: Bio-organic chemistry
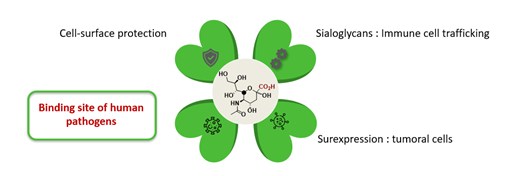
Sialic acids (SA) are a class of α-keto acid sugars with a nine-carbon backbone composed by more than 80 natural members. The most common member - found in mammalian cells - is N-acetylneuraminic acid (Neu5Ac or NANA). It has multiple biological functions: protection of cell surface, surexpression in tumoral cells, immune cells trafficking, and binding site of human pathogens.[1] Indeed, the Influenza viruses and the SARS-CoV-2 has selective affinity to sialic acid derivatives on cell host surface: this first contact will enable viruses entry in the cell host. [2]
Influenza A subtype H1N1 caused two pandemics – Spanish flu (1918) and Swine flu (2009) - which resulted in 20-50 Million and 20 000-500 000 deaths respectively.[3]More recently, on December 2019, SARS-CoV-2 appeared and forced governments to implement drastic measures. In the beginning of the infection, this virus caused 3.5 Million deaths. Currently, the number of deaths had increased to 6.9 Million.[4]
 |
Influenza A subtype H1N1 caused two pandemics – Spanish flu (1918) and Swine flu (2009) - which resulted in 20-50 Million and 20 000-500 000 deaths respectively.[3]More recently, on December 2019, SARS-CoV-2 appeared and forced governments to implement drastic measures. In the beginning of the infection, this virus caused 3.5 Million deaths. Currently, the number of deaths had increased to 6.9 Million.[4]
The main objective of this PhD project is to develop new methodologies to synthesize borylated carbohydrates and glycals. The targeted molecules will be assayed as antiviral molecules.
 |
[1] a) E. A. Visser, et al., J. Biol. Chem. 2021, 297, 100906; b) D. Aquino, R. Wong, R. U. Margolis, R. K. Margolis, FEBS Letters 1980, 112, 195-198; c) R. P. McEver, K. L. Moore, R. D. Cummings, J. Biol. Chem. 1995, 270, 11025-11028.
[2] a) S. J. L. Petitjean, et al., Nat. Commun. 2022, 13, 2564; b) S. J. Gamblin, et al., Cold Spring Harb. Perspect. Med. 2021, 11.
[3] P. Spreeuwenberg, M. Kroneman, J. Paget, Am. J. Epidemiol. 2018, 187, 2561-2567.
[4] WHO, WHO Coronavirus (COVID-19) Dashboard 2023, https://covid19.who.int/.
The team
 |
 |
 |
| See the members of the team |
Publications
EXO-Sugar-MAP | An ARC project
 Funded by the Federation Wallonie-Bruxelles (FWB), ARC projects are Concerted Research Action projects that aim at developing university or inter-university centres of excellence in fundamental research axes and, where possible, that carry out basic and applied research in an integrated manner and aim to make economic and social use of research results. They are awarded based on academic excellence of the applicants, the added value of each research group to achieve goals of research project, complementary skills of research teams and the methodology of proposed research program. They typically last for 4 to 5 years. In case of inter-university project, each team is financely supported by its own institution
Funded by the Federation Wallonie-Bruxelles (FWB), ARC projects are Concerted Research Action projects that aim at developing university or inter-university centres of excellence in fundamental research axes and, where possible, that carry out basic and applied research in an integrated manner and aim to make economic and social use of research results. They are awarded based on academic excellence of the applicants, the added value of each research group to achieve goals of research project, complementary skills of research teams and the methodology of proposed research program. They typically last for 4 to 5 years. In case of inter-university project, each team is financely supported by its own institution
More information on the FWB website

